Publications
Original Papers
49.
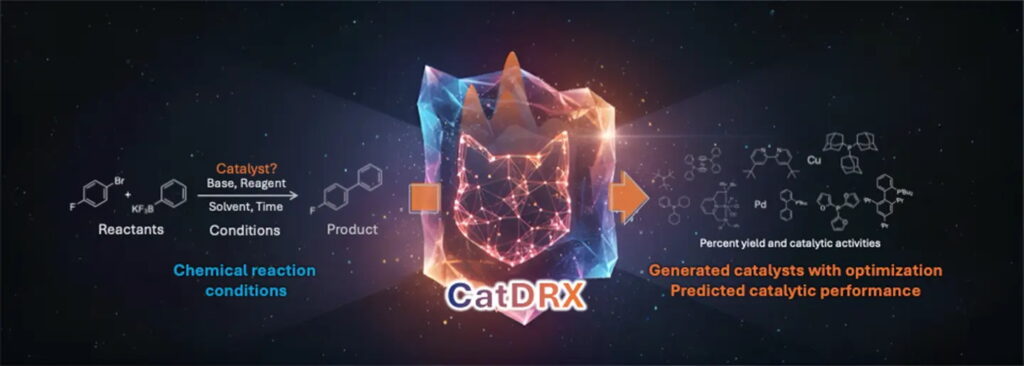
Reaction-conditioned generative model for catalyst design and optimization with CatDRX
Apakorn Kengkanna, Yuta Kikuchi, Takashi Niwa, Masahito Ohue*
Commun. Chem. 2025, 8, 314.
doi: 10.1038/s42004-025-01732-7 (open access)
Science Tokyo Press Release / Kyushu Univ. Press Release (in Japanese)
ホームページお知らせ
48.
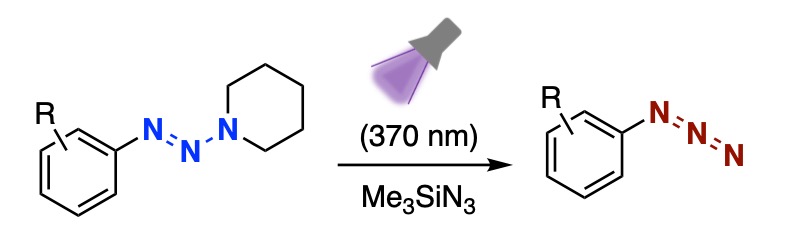
Photoirradiation-Promoted ipso-Azidation of 1-Aryltriazenes
Takashi Niwa,* Yamato Ezo, Satoshi Fukuda, Kenji Watanabe, Isao Kii, Takamitsu Hosoya*
Org. Lett. 2025, 27, 13192–13197.
doi: 10.1021/acs.orglett.5c03938 [Supplemantary Cover Figure]
ホームページお知らせ(光アジド化論文が採択、公開されました!)

47.
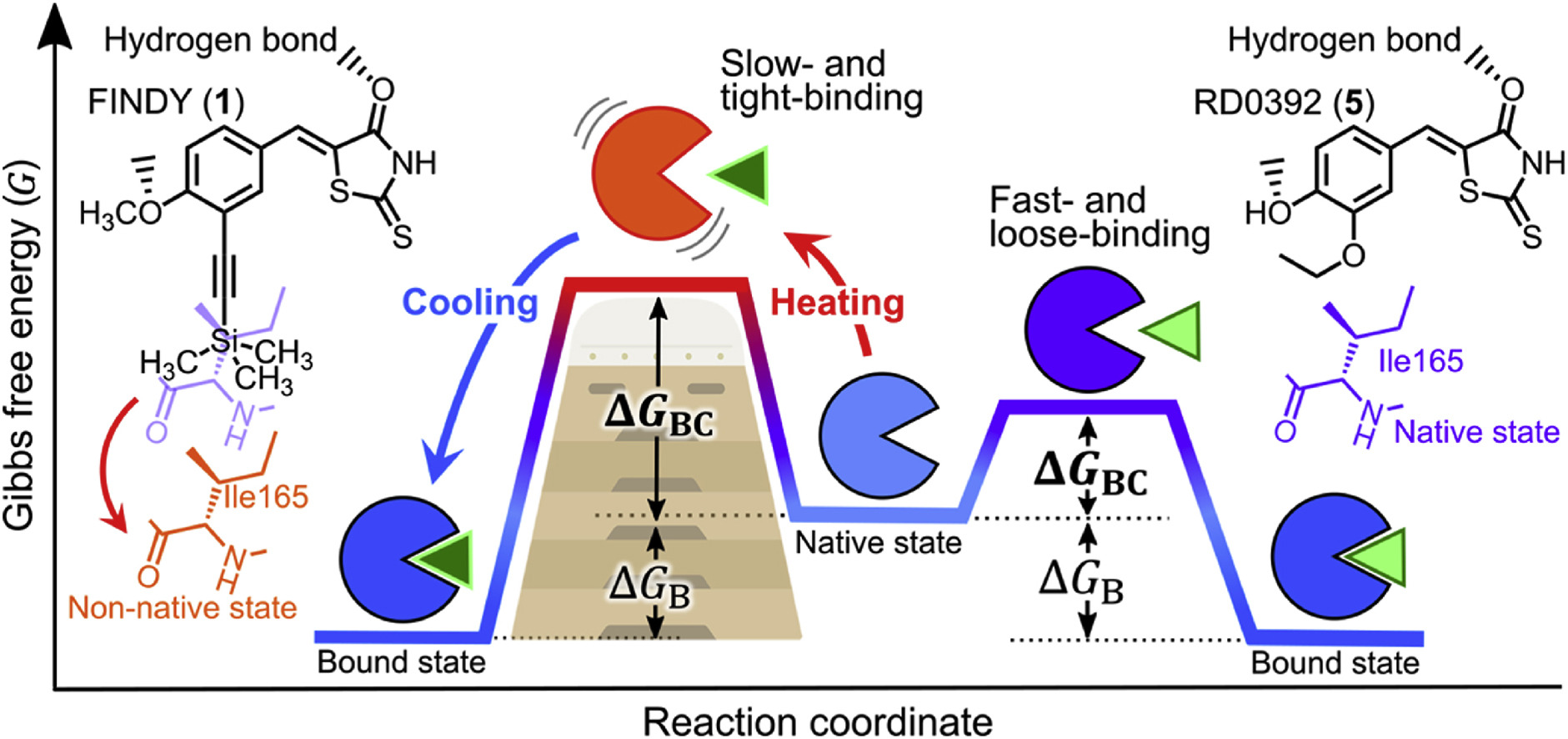
Temperature vaulting: A method for screening of slow- and tight-binding inhibitors that selectively target kinases in their non-native state
Sora Suzuki, Koji Umezawa, Gaku Furuie, Masaki Kikuchi, Daichi G. M. Nakamura, Nanae Fukahori, Ninako Kimura, Masato Yamakawa, Takashi Niwa, Takashi Umehara, Takamitsu Hosoya, Isao Kii*
Eur. J. Med. Chem. 2025, 295, 117789.
doi: 10.1016/j.ejmech.2025.117789
46.
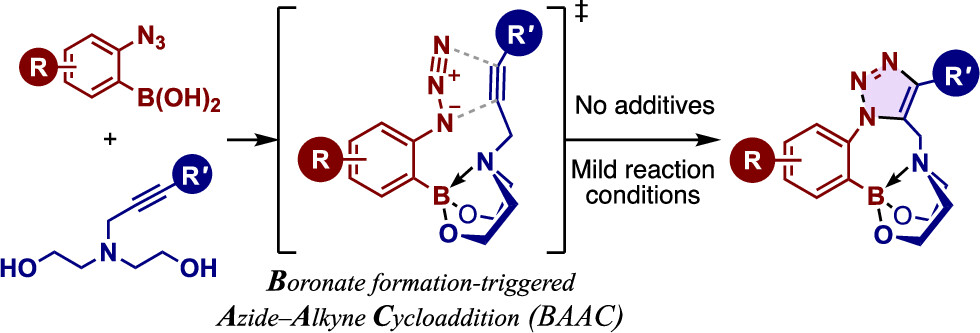
Boronate Formation-Triggered Azide–Alkyne Cycloaddition
Junpei Taguchi*, Yohei Ohata, Honoka Akimoto, Hitomi Tabuchi, Kazunobu Igawa, Katsuhiro Tomooka, Takashi Niwa, Takamitsu Hosoya*
Org. Lett. 2025, 27, 4428–4433.
doi: 10.1021/acs.orglett.5c00732
45.
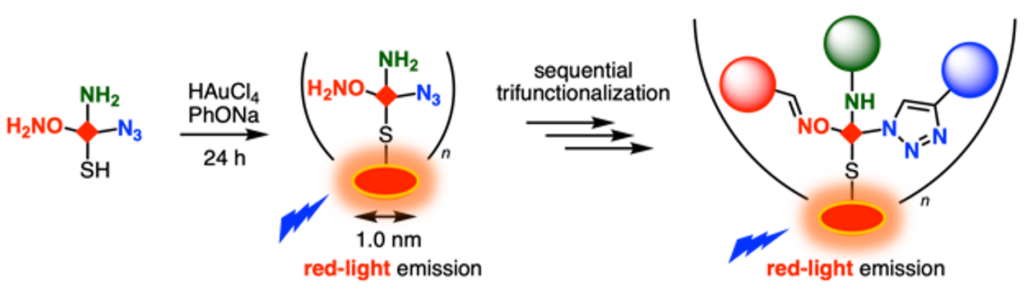
Red-light emitting orthogonally trireactive gold nanoclusters for the synthesis of multifunctionalized nanomaterials
Kenji Watanabe*, Yuta Uetake*, Machi Hata, Anna Kuwano, Riko Yamamoto, Yasutomo Yamamoto, Masahito Kodera, Hiroaki Kitagishi, Takashi Niwa, Takamitsu Hosoya*
Small 2024, 21, 2408747.
doi: 10.1002/smll.202408747 (open access)
44.
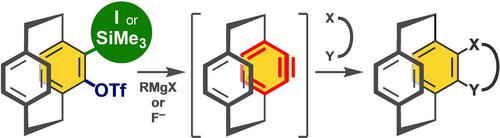
Revisiting the Synthetic Utility of 4,5-Dehydro[2.2]paracyclophane
Jumpei Taguchi, Yuta Omoto, Konami Uto, Hitomi Tabuchi, Hidehiro Uekusa, Takashi Niwa, Takamitsu Hosoya*
Adv. Synth. Catal. 2024, early view.
doi: 10.1002/adsc.202400986
43.
Clickable bisreactive small gold nanoclusters for preparing multifunctionalized nanomaterials: application to photouncaging of an anticancer molecule
Kenji Watanabe*, Qiyue Mao, Zhouen Zhang, Machi Hata, Masahito Kodera, Hiroaki Kitagishi,
Takashi Niwa, Takamitsu Hosoya*
Chem. Sci. 2024, 15, 1402–1408.
doi: 10.1039/D3SC04365G

42.
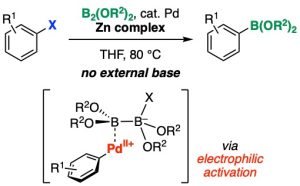
Palladium-Catalyzed ipso-Borylation of Aryl Halides Promoted by Lewis Acid-Mediated Electrophilic Activation of Aryl(halo)palladium(II) Complex
Takashi Niwa*, Tadashi Takimoto, Yuki Sakata, Takamitsu Hosoya*
Org. Lett. 2023, 25, 8173–8177.
doi: 10.1021/acs.orglett.3c03531
41.
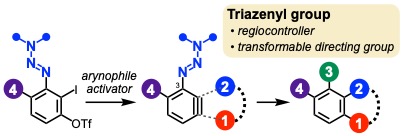
Synthesis of Multisubstituted Aromatics via 3–Triazenylarynes
Jumpei Taguchi, Takumi Okuyama, Satomi Tomita, Takashi Niwa, Takamitsu Hosoya*
Org. Lett. 2023, 25, 7030–7034.
doi: 10.1021/acs.orglett.3c02615
40.
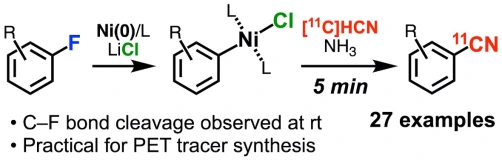
11C-Cyanation of Aryl Fluorides via Nickel and Lithium Chloride-Mediated C–F Bond Activation
Zhouen Zhang*, Takashi Niwa, Kenji Watanabe, Takamitsu Hosoya*
Angew. Chem. Int. Ed. 2023, 62, e202302956.
doi: 10.1002/anie.202302956
39.
Acute nicotine exposure blocks aromatase in the limbic brain of healthy women: A [11C]cetrozole PET study
Manon Dubol, Jana Immenschuh, My Jonasson, Kayo Takahashi, Takashi Niwa, Takamitsu Hosoya, Sara Roslin, Johan Wikström, Gunnar Antoni, Yasuyoshi Watanabe, Mark Lubberink, Anat Biegon, Inger Sundström-Promaa, Erika Comasco*
Compr. Psychiatry 2023, 123, 152381.
doi: 10.1016/j.comppsych.2023.152381
38.
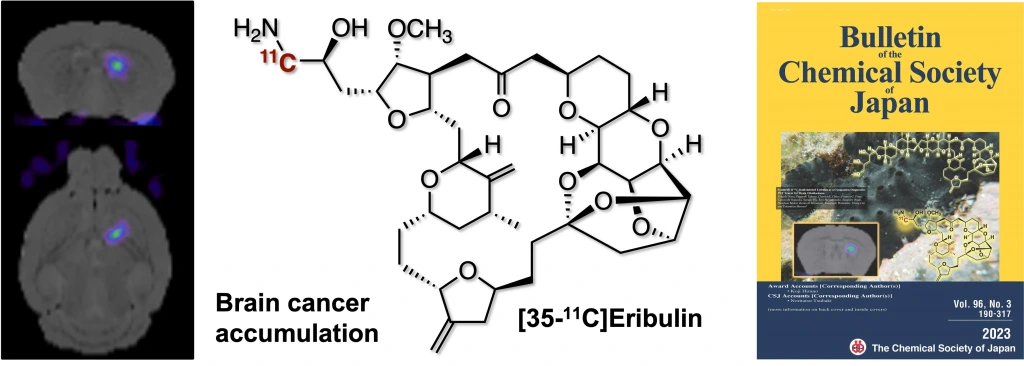
Synthesis of 11C-Radiolabeled Eribulin as a Companion Diagnostics PET Tracer for Brain Glioblastoma
Takashi Niwa, Tsuyoshi Tahara, Charles E. Chase, Francis G. Fang, Takayoshi Nakaoka, Satsuki Irie, Emi Hayashinaka, Yasuhiro Wada, Hidefumi Mukai, Kenkichi Masutomi, Yasuyoshi Watanabe, Yilong Cui, Takamitsu Hosoya*
Bull. Chem. Soc. Jpn. 2023, 96, 283–290.
doi: 10.1246/bcsj.20220335 (open access)
Press release(極めて複雑な合成医薬分子に短寿命核種を導入)[BCSJ Award Article]
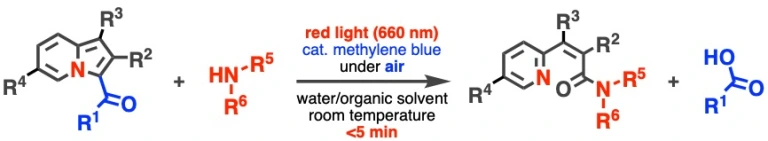
37.
Red light-induced conjugation of amines through amide bond formation triggered via photooxidation of 3-acylindolizines
Kenji Watanabe*, Asuka Kuratsu, Daisuke Hashizume, Takashi Niwa, Takamitsu Hosoya
Commun. Chem. 2022, 5, 91.
doi: 10.1038/s42004-022-00712-5
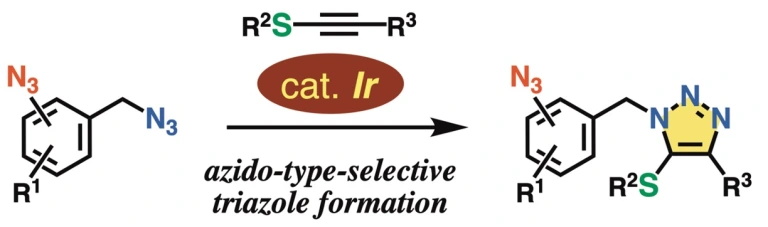
36.
Azido-Type-Selective Triazole Formation by Iridium-Catalyzed Cycloaddition with Thioalkyne
Kazuya Sugiyama, Yuki Sakata, Takashi Niwa, Suguru Yoshida, Takamitsu Hosoya*
Chem. Commun. 2022, 58, 6235–6238.
doi: 10.1039/d2cc01739c
35.
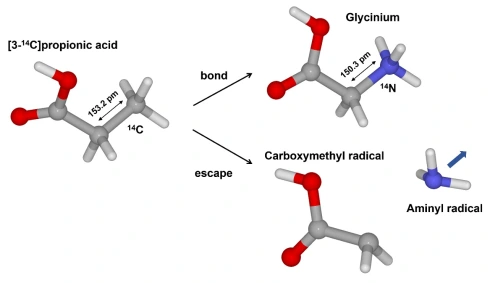
Computational study for amino acid production from carboxylic acid via 14C β-decay
Tomonori Fukuchi*, Takashi Niwa, Takamitsu Hosoya, Yasuyoshi Watanabe
J. Phys. Soc. Jpn. 2022, 91, 064301.
doi: 10.7566/JPSJ.91.064301
Press release(窒素がなくてもアミノ酸はできる!)
34.
Expression and purification of DYRK1A kinase domain in complex with its folding intermediate-selective inhibitor FINDY
Ninako Kimura, Kanako Saito, Takashi Niwa, Masato Yamakawa, Shota Igaue, Junko Ohkanda, Takamitsu Hosoya, Isao Kii*
Protein Expr. Purif. 2022, 195–196, 106089.
doi: 10.1016/j.pep.2022.106089
33.
Clinical evaluation of [18F]pitavastatin for quantitative analysis of hepatobiliary transporter activity
Takayoshi Nakaoka, Ken-ichi Kaneko, Satsuki Irie, Aya Mawatari, Ami Igesaka, Yuta Uetake, Hidenori Ochiai, Takashi Niwa, Emi Yamano, Yasuhiro Wada, Masaaki Tanaka, Kohei Kotani, Hideki Kawahata, Joji Kawabe, Yukio Miki, Hisashi Doi, Takamitsu Hosoya, Kazuya Maeda, Hiroyuki Kusuhara, Yuichi Sugiyama, Yasuyoshi Watanabe*
Drug Metab. Pharmacokinet. 2022, 44, 100449.
doi: 10.1016/j.dmpk.2022.100449
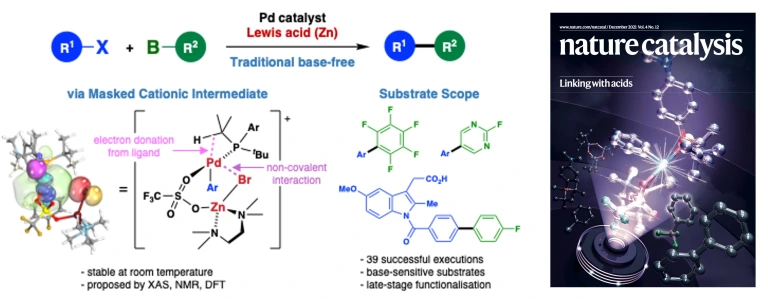
32.
Lewis acid-mediated Suzuki–Miyaura Cross-Coupling Reaction
Takashi Niwa*, Yuta Uetake*, Motoyuki Isoda, Tadashi Takimoto, Miki Nakaoka, Daisuke Hashizume, Hidehiro Sakurai, Takamitsu Hosoya
Nat. Catal. 2021, 4, 1080–1088.
doi: 10.1038/s41929-021-00719-6 (open access)
Deposited to ChemRxiv, 2021, DOI: 10.33774/chemrxiv-2021-kmfr5-v3
Press release(塩基の代わりに酸を使うクロスカップリング反応)
Highlighted in Synfacts 2022, 18, 392.
31.
Structure-activity relationship for the folding intermediate-selective inhibition of DYRK1A
Yuka Miyazaki, Masaki Kikuchi, Koji Umezawa, Aurelie Descamps, Daichi Nakamura, Gaku Furuie, Tomoe Sumida, Kanako Saito, Ninako Kimura, Takashi Niwa, Yuto Sumida, Takashi Umehara, Takamitsu Hosoya, Isao Kii*
Eur. J. Med. Chem. 2021, 86, 113948.
doi: 10.1016/j.ejmech.2021.113948
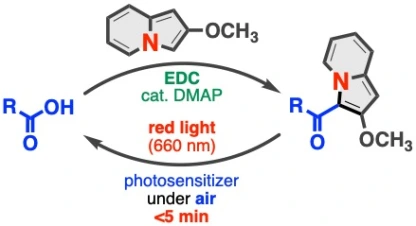
30.
Direct 3-Acylation of Indolizines by Carboxylic Acids for the Practical Synthesis of Red Light-Releasable Caged Carboxylic Acids
Kenji Watanabe*, Nodoka Terao, Takashi Niwa, Takamitsu Hosoya*
J. Org. Chem. 2021, 86, 11822–11834.
doi: 10.1021/acs.joc.1c01244
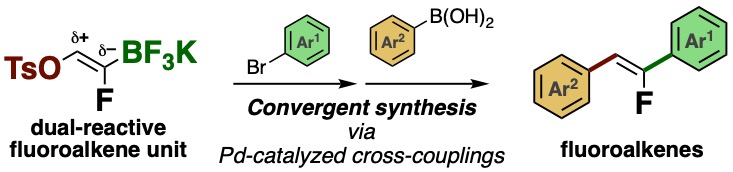
29.
Convergent Synthesis of Fluoroalkenes Using a Dual-Reactive Unit
Motoyuki Isoda, Yuta Uetake, Tadashi Takimoto, Junpei Tsuda, Takamitsu Hosoya*, Takashi Niwa*
J. Org. Chem. 2021, 86, 1622–1632.
doi: 10.1021/acs.joc.0c02474
28.
Quantification of aromatase binding in the female human brain using [11C]cetrozole PET
My Jonasson, Patrik Nordeman, Jonas Eriksson, Helena Wilking, Johan Wikström, Kayo Takahashi, Takashi Niwa, Takamitsu Hosoya, Yasuyoshi Watanabe, Gunnar Antoni, Inger Sundström Poromaa, Mark Lubberink, Erika Comasco*
J. Neurosci. Res. 2020, 98, 2208–2218.
doi: 10.1002/jnr.24707
27.
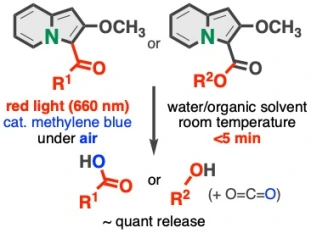
Indolizines Enabling Rapid Uncaging of Alcohols and Carboxylic Acids by Red Light-Induced Photooxidation
Kenji Watanabe*, Nodoka Terao, Isao Kii, Reiko Nakagawa, Takashi Niwa, Takamitsu Hosoya*
Org. Lett. 2020, 22, 5434–5438.
doi: 10.1021/acs.orglett.0c01799
26.
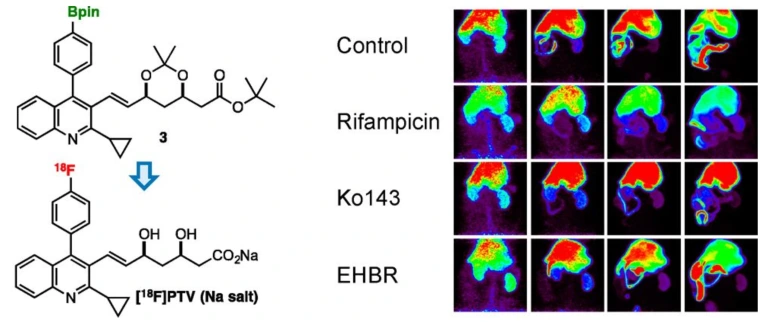
Practical synthesis of [18F]pitavastatin and evaluation of hepatobiliary transport activity in rats by positron emission tomography
Takayoshi Nakaoka, Yuta Uetake, Ken-ichi Kaneko, Takashi Niwa, Hidenori Ochiai, Satsuki Irie, Yoshie Suezaki, Natsumi Otsuka, Emi Hayashinaka, Yasuhiro Wada, Yilong Cui, Kazuya Maeda, Hiroyuki Kusuhara, Yuichi Sugiyama, Takamitsu Hosoya, Yasuyoshi Watanabe*
Mol. Pharmaceutics 2020, 17, 1884–1898.
doi: 10.1021/acs.molpharmaceut.9b01284
25.
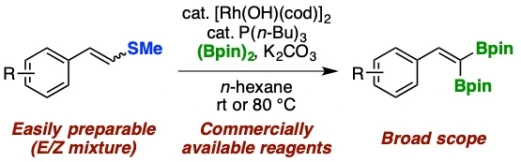
Synthesis of (2,2-Diborylvinyl)arenes by Rhodium-Catalyzed Desulfanylative gem-Diborylation of 2-Arylvinyl Sulfides
Yuta Uetake, Motoyuki Isoda, Takashi Niwa*, Takamitsu Hosoya*
Org. Lett. 2019, 21, 4933–4938.
doi: 10.1021/acs.orglett.9b01253
24.
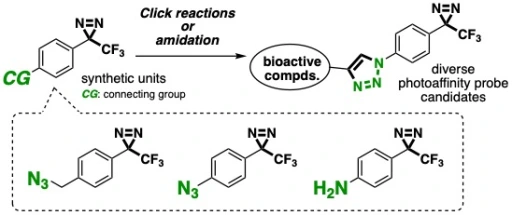
Divergent synthesis of photoaffinity probe candidates by click reactions of azido-substituted aryltrifluoromethyldiazirines
Kenji Watanabe, Junpei Tsuda, Hidenori Ochiai, Takashi Niwa, Takamitsu Hosoya*
Heterocycles 2019, 99, 1366–1387.
doi: 10.3987/COM-18-S(F)76
23.
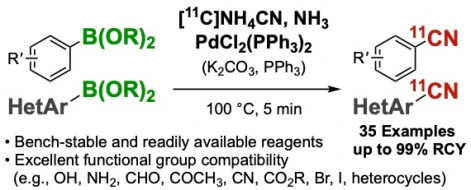
Palladium(II)-mediated rapid 11C-cyanation of (hetero)arylborons
Zhouen Zhang*, Takashi Niwa, Yasuyoshi Watanabe, Takamitsu Hosoya*
Org. Biomol. Chem. 2018, 16, 7711–7716.
doi: 10.1039/C8OB02049C
22.
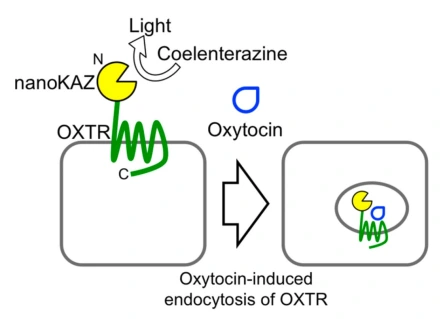
Quantification of receptor activation by oxytocin and vasopressin in endocytosis-coupled bioluminescence reduction assay using nanoKAZ
Isao Kii*, Shino Hirahara-Owada, Masataka Yamaguchi, Takashi Niwa, Yuka Koike, Rie Sonamoto, Harumi Ito, Kayo Takahashi, Chihiro Yokoyama, Takuya Hayashi, Takamitsu Hosoya, Yasuyoshi Watanabe
Anal. Biochem. 2018, 549, 174–183.
doi: 10.1016/j.ab.2018.04.001
21.
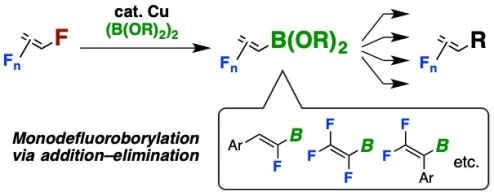
Copper-Catalyzed Regioselective Monodefluoroborylation of Polyfluoroalkenes en Route to Diverse Fluoroalkenes
Hironobu Sakaguchi,§ Yuta Uetake,§ Masato Ohashi, Takashi Niwa*, Sensuke Ogoshi*, Takamitsu Hosoya* (§H.S. and Y.U. contributed equally)
J. Am. Chem. Soc. 2017, 139, 12855–12862.
doi: 10.1021/jacs.7b08343
Press release(フルオロアルケンの簡便合成を実現)
20.
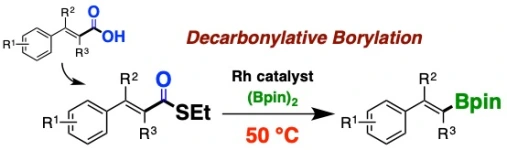
Facile Transformation of α,β-Unsaturated Carboxylic Acids to Alkenylboronic Esters via Rhodium-Catalyzed Decarboxylative Borylation of α,β-Unsaturated Thioesters
Takashi Niwa*, Hidenori Ochiai, Motoyuki Isoda, Takamitsu Hosoya*
Chem. Lett. 2017, 46, 1315–1318.
doi: 10.1246/cl.170549 (open access, selected as Editor’s Choice)
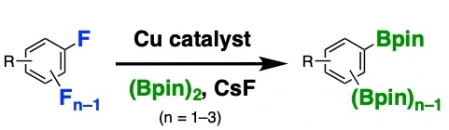
19.
Copper-Catalyzed ipso-Borylation of Fluoroarenes
Takashi Niwa*, Hidenori Ochiai, Takamitsu Hosoya*
ACS Catal. 2017, 7, 4535–4541.
doi: 10.1021/acscatal.7b01448 (open access)
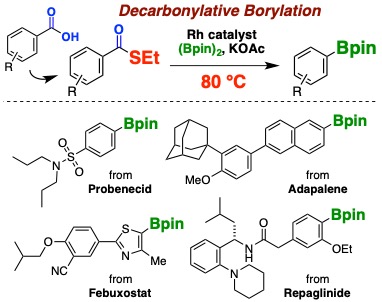
18.
Rhodium-Catalyzed Decarbonylative Borylation of Aromatic Thioesters for Facile Diversification of Aromatic Carboxylic Acids
Hidenori Ochiai, Yuta Uetake, Takashi Niwa*, Takamitsu Hosoya*
Angew. Chem., Int. Ed. 2017, 56, 2482–2486.
doi: 10.1002/anie.201611974 (open access)
Press release(カルボン酸の自在変換法を開発)
Highlighted in Synfacts 2017, 13, 407.
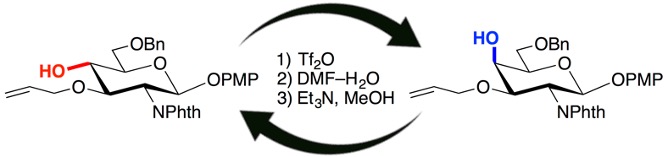
17.
Stereoinversion of Stereocongested Carbocyclic Alcohols via Triflylation and Subsequent Treatment of Aqueous N,N-Dimethylformamide
Hidenori Ochiai, Takashi Niwa, Takamitsu Hosoya*
Org. Lett. 2016, 18, 5982–5985.
doi: 10.1021/acs.orglett.6b02675
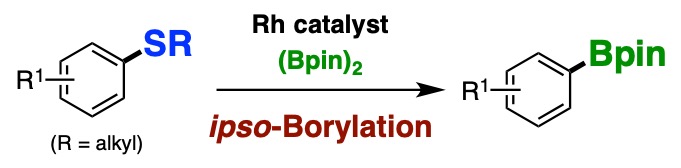
16.
Rhodium-Catalyzed ipso-Borylation of Alkylthioarenes via C–S Bond Cleavage
Yuta Uetake, Takashi Niwa, Takamitsu Hosoya*
Org. Lett. 2016, 18, 2758–2761.
doi: 10.1021/acs.orglett.6b01250
15.
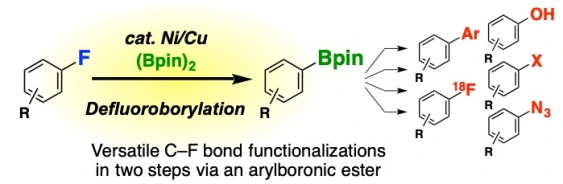
Ni/Cu-Catalyzed Defluoroborylation of Fluoroarenes for Diverse C–F Bond Functionalizations
Takashi Niwa*, Hidenori Ochiai, Yasuyoshi Watanabe, Takamitsu Hosoya*
J. Am. Chem. Soc. 2015, 137, 14313–14318.
doi: 10.1021/jacs.5b10119
Press release(薬剤分子の新たな化学変換法)
14.
Highly enantioselective catalytic asymmetric Mukaiyama–Michael reactions of cyclic α-alkylidene β-oxo imides
Harufumi Oyama, Kohei Orimoto, Takashi Niwa, Masahisa Nakada*
Tetrahedron Asymmetry 2015, 26, 262–270.
doi: 10.1016/j.tetasy.2015.01.012
13.
Synthesis and characterization of a new C2-symmetric chiral tridentate NHC-ligand-coordinated Cr(III) complex
Yuta Uetake, Takashi Niwa, Masahisa Nakada*
Tetrahedron Asymmetry 2015, 26, 158–162.
doi: 10.1016/j.tetasy.2015.01.003
12.
Synthesis of cycloalkanone-fused cyclopropanes by Au(I)-catalyzed oxidative ene-yne cyclization
Yuta Uetake, Takashi Niwa, Masahisa Nakada*
Tetrahedron Lett. 2014, 55, 6847–6850.
doi: 10.1016/j.tetlet.2014.10.084
11.
Preparations of Imides via the Palladium-Catalyzed Coupling Reaction of Organostannanes with Methyl N-[Methoxy(methylthio)methylene]carbamate
Kohei Orimoto, Takuhei Tomizawa, Yuki Namera, Harufumi Oyama, Takashi Niwa, Masahisa Nakada*
Heterocycles 2013, 87, 827–840.
doi: 10.3987/COM-13-12662
10.
Catalytic Asymmetric [4+2] Cycloadditions and Hosomi–Sakurai Reactions of α-Alkylidene β-Keto Imides
Kohei Orimoto, Harufumi Oyama, Yuki Namera, Takashi Niwa, Masahisa Nakada*
Org. Lett. 2013, 15, 768–771.
doi: 10.1021/ol303381c
9.
Preparation of Imides via the Palladium-Catalyzed Coupling Reaction of Organoborons with Methyl N-[Methoxy(methylthio)methylene]carbamate as a One-Carbon Elongation Reaction
Takuhei Tomizawa, Kohei Orimoto, Takashi Niwa, Masahisa Nakada*
Org. Lett. 2012, 14, 6294–6297.
doi: 10.1021/ol303062a
8.
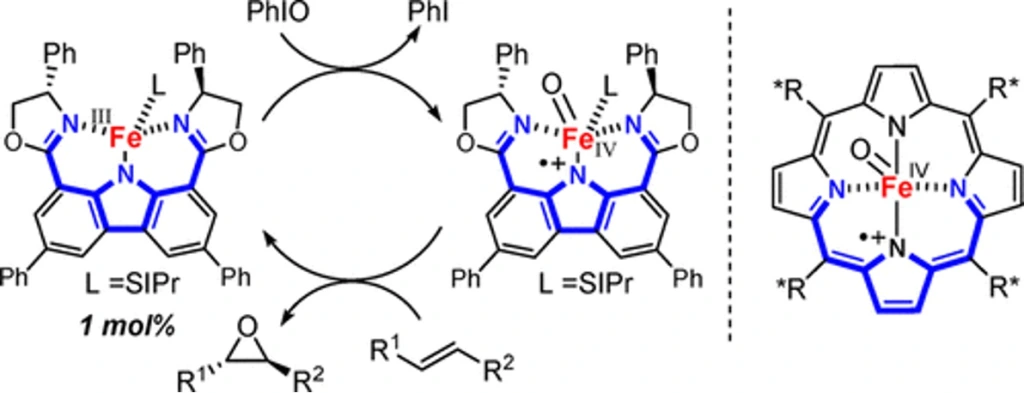
A Non-Heme Iron(III) Complex with Porphyrin-like Properties That Catalyzes Asymmetric Epoxidation
Takashi Niwa*, Masahisa Nakada*
J. Am. Chem. Soc. 2012, 134, 13538–13541.
doi: 10.1021/ja304219s
7.
Pd-Catalyzed reductive cleavage of alkyl aryl sulfides with triethylsilane that is accelerated by trialkylsilyl chloride
Takehiko Matsumura, Takashi Niwa, Masahisa Nakada*
Tetrahedron Lett. 2012, 53, 4313–4316.
doi: 10.1016/j.tetlet.2012.06.002
6.
Palladium-Catalyzed (N-Oxido-2-pyridinyl)methyl Transfer from 2-(2-Hydroxyalkyl)pyridine N-Oxide to Aryl Halides by β-Carbon Elimination
Takafumi Suehiro, Takashi Niwa, Hideki Yorimitsu*, Koichiro Oshima*
Chem. Asian J. 2009, 4, 1217–1220.
doi: 10.1002/asia.200900151
5.
Carbon–Carbon Bond Formations at the Benzylic Positions of N-Benzylxanthone and N-Benzyldi-1-naphthyl Ketone Imine
Takashi Niwa, Takafumi Suehiro, Hideki Yorimitsu*, Koichiro Oshima*
Tetrahedron 2009, 65, 5125–5131.
doi: 10.1016/j.tet.2009.02.034
4.
Palladium-Catalyzed Benzylic Direct Arylation of Benzyl Sulfone
Takashi Niwa, Hideki Yorimitsu*, Koichiro Oshima*
Tetrahedron 2009, 65, 1971–1976.
doi: 10.1016/j.tet.2009.01.030
3.
Palladium-Catalyzed Benzylic Arylation of N-Benzylxanthone Imine
Takashi Niwa, Hideki Yorimitsu*, Koichiro Oshima*
Org. Lett. 2008, 10, 4689–4691.
doi: 10.1021/ol802070d
2.
Palladium-Catalyzed Direct Arylation of Aryl(azaaryl)methanes with Aryl Halides Providing Triarylmethanes
Takashi Niwa, Hideki Yorimitsu*, Koichiro Oshima*
Org. Lett. 2007, 9, 2373–2375.
doi: 10.1021/ol0708119
1.
Palladium-Catalyzed 2-Pyridylmethyl Transfer from 2-(2-Pyridyl)ethanol Derivatives to Organic Halides by Chelation-Assisted Cleavage of Unstrained sp3C–sp3C Bonds
Takashi Niwa, Hideki Yorimitsu*, Koichiro Oshima*
Angew. Chem., Int. Ed. 2007, 46, 2643–2645.
doi: 10.1002/anie.200604472
Others (Mainly written in Japanese)
17. 総合論文
PETプローブ開発を指向した分子設計と合成戦略
丹羽 節, 細谷孝充
有機合成化学協会誌, 2024, 82(5), 433–449.
16. 解説
複雑な構造を有するPETプローブの開発
丹羽 節, 細谷孝充
アイソトープニュース(公益社団法人日本アイソトープ協会), 2024, 792(4), 2–6.
15. 解説
脳膠芽腫のコンパニオン診断用PETトレーサーとしての[11C]エリブリンの合成
丹羽 節, 田原 強, Charles E. Chase, Francis G. Fang, 中岡貴義, 入江さつき, 林中恵美, 和田康弘, 向井英史, 増富健吉, 渡辺恭良, 崔 翼龍, 細谷孝充
JSMI Report(日本分子イメージング学会学会誌), 2024, 17(1), 30–33.
14. 著書(分担)
clickable光親和性標識プローブを用いた標的分子同定
丹羽 節, 喜井 勲, 細谷孝充
実験医学増刊:あなたのラボから薬を生み出す アカデミア創薬の実践 All JAPAN体制の先端技術支援を利用した創薬の最前線化学, 善光龍哉, 辻川和丈 編集, 2024, 42(2), 205–211.
13. 解説
塩基を添加しないクロスカップリング反応 –– 大型放射光施設を活用した反応機構の解析
丹羽節, 植竹裕太, 細谷孝充
化学(化学同人, 解説) 2022, 77(5), 12–16.
12. Review
Molecular Renovation Strategy for Expeditious Synthesis of Molecular Probes
Takashi Niwa*, Takamitsu Hosoya*
Bull. Chem. Soc. Jpn. 2020, 93, 230–248.
doi: 10.1246/bcsj.20190310
11. Review
芳香族フッ化物の脱フッ素ホウ素化
丹羽 節, 細谷孝充
有機合成化学協会誌 2019, 77, 883–894.
doi: 10.1246/bcsj.20190310
10. 解説
光触媒で加速する脱フッ素ホウ素化
丹羽 節
Organometallic News(有機金属ハイライト) 2019, No. 2, 79.
9. 著書(分担)
TFE, HFP, CTFEなどの安価な市販のフッ素原料を用いた合成
丹羽 節
ファインケミカルシリーズ 有機フッ素化合物の最新動向, 今野 勉 監修(シーエムシー出版), pp 107–116 (2018).
8. 解説
分子をつくり変える –PETイメージングへの応用に向けて–
丹羽 節, 細谷孝充
現代化学(東京化学同人), 2018, 568(7), 42–46.
7. Review
医薬品をPETプローブに作り変える分子リノベーション技術の開発
丹羽 節, 細谷孝充
アイソトープニュース(公益社団法人日本アイソトープ協会), 2017, 753(10), 8–13.
6. Essay
自然界のルールを破った、その先に
丹羽 節
産経新聞2017年5月25日朝刊関西地域面 科学の中身 理化学研究所 関西編
5. Review
低分子PETプローブの開発
丹羽 節, 細谷孝充
日本レーザー医学会誌, 2017, 37(4), 465–472.
4. 解説
[18F]トリフルオロメチル化:1つだけ異なるフッ素を導入する方法
丹羽 節
ファルマシア((財)日本薬学会、トピックス), 2014, 50(7), 691.
3. 解説
メタノールを有機化学の材料に
丹羽 節
化学((株)化学同人、Review of Chemistry in 2011、注目の論文), 2011, 66(8), 64–65.
2. 解説
可視光で励起する遷移金属錯体を用いた有機合成反応
丹羽 節
有機合成化学協会誌(Review de Debut), 2010, 68(12), 1307–1308.
1. 解説
ハーバード大学(Ritter研)での留学生活
丹羽 節
OM News(近畿化学協会編、海外研究室レポート), 2010, No. 2, 50–51.



