Research
Organic reactions are often used to synthesize artificially designed molecules, but they are also a natural process that occurs constantly in all environments, including in living organisms. By deepening and utilization of organic reaction chemistry, our group is engaged in the creation of molecules that will revolutionize drug discovery, medicine, and life science research, as well as create new methodologies that contribute to the visualization and control of biological functions.
(1) Developing “new” organic reactions to realize unimaginable transformations
We will realize chemical transformations that are currently considered impossible by developing new chemical processes. We will explore organic reaction chemistry as a basic science by pursuing newly discovered reaction mechanisms and further generalization.
Molecular Renovation Technology for Rapid Functionalization of Biologically Active Compounds
In drug discovery and life science research, elucidating the mechanisms of bioactive compounds is an important issue. For this purpose, using molecular probes that are functionalized by modifying the original molecules is effective. However, in general, preparing such molecules requires time-consuming and laboring work. This is due to the fact that bioactive compounds originally have complex chemical structures and, depending on the position of the modification, the synthesis method of the original compound cannot be followed and thus a new synthetic route is required.
To address the practical issue, we anticipated that regioselective borylation of bioactive compounds and the following versatile transformation provides molecular probes with a desired function in only two steps in principle.1
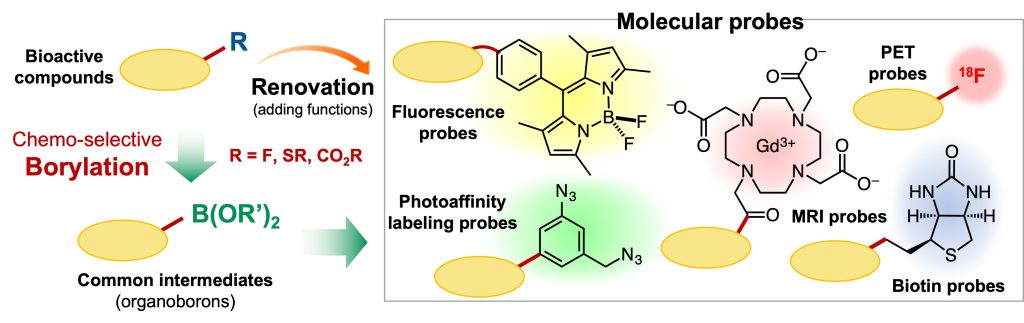
Based on this idea, we focused on aromatic fluorides, which are widely found in pharmaceuticals, and developed a borylation reaction that involves the cleavage of a carbon-fluorine bond.2-4 This transformation involves a contradiction; the most stable (less reactive) aromatic carbon-fluorine bond cleaves to afford organoborons bearing a reactive carbon-boron bond. Combining this method and 18F-radiolabeling reaction, we succeeded in renovating a statin derivative, a drug for hyperlipidemia, into an 18F-labeled PET probe within only two steps.
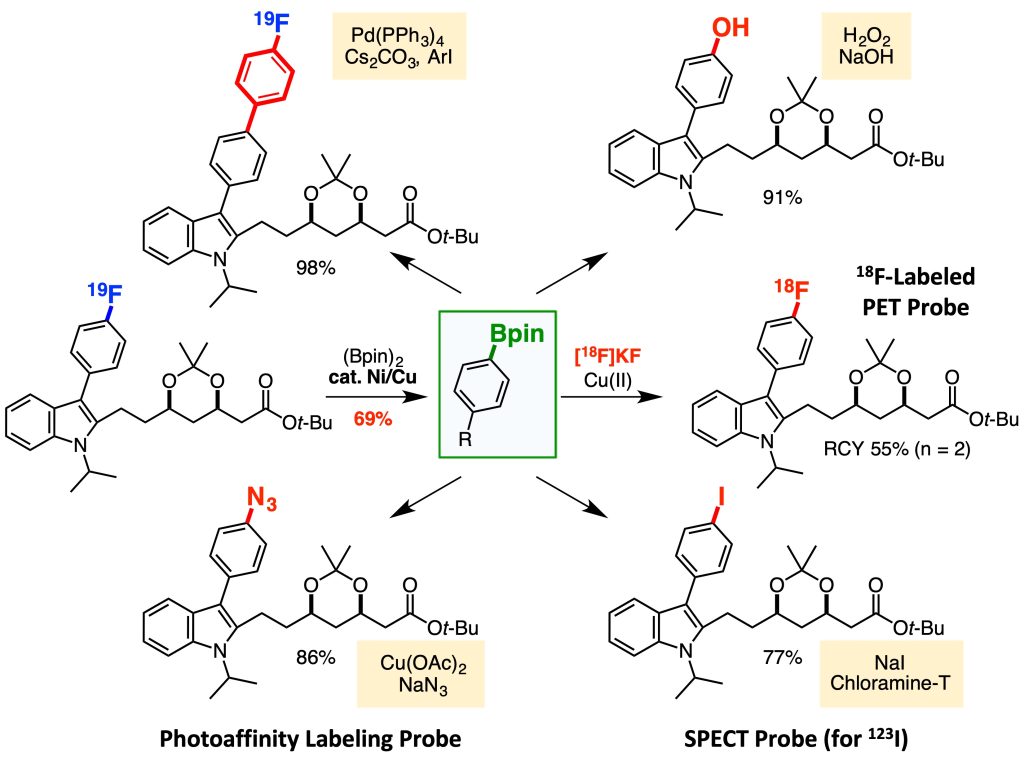
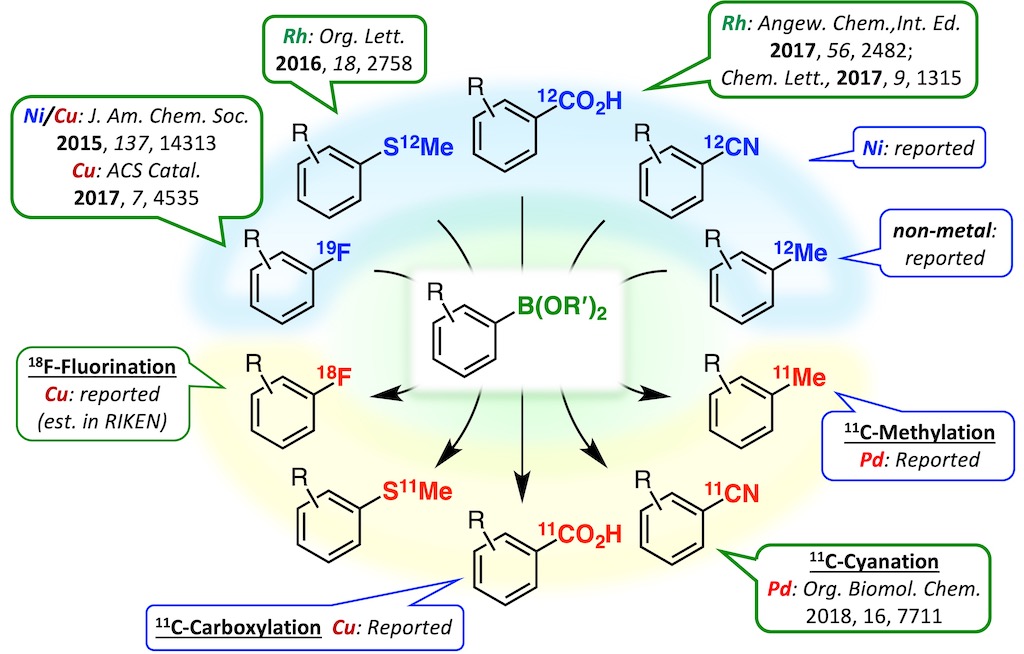
This result prompted us to develop methods for converting various functional groups to boryl groups.5-7 Further extensions of this technology will contribute to accelerating the expeditious creation of molecular probes based on direct modifications of bioactive compounds.
1. Review: Bull. Chem. Soc. Jpn. 2020, 93, 230–248.
2. J. Am. Chem. Soc. 2015, 137, 14313–14318.
3. ACS Catal. 2017, 7, 4535–4541.
4. Review: J. Synth. Org. Chem. Jpn. 2019, 77, 883–894.
5. Org. Lett. 2016, 18, 2758–2761.
6. Angew. Chem., Int. Ed. 2017, 56, 2482–2486.
7. Chem. Lett. 2017, 46, 1315–1318.
2) Precise modification of complex molecules using a variety of external stimuli
Chemical transformations involve the cleavage of covalent bonds and thus require reactive chemical species with large potential energies. However, treatment of complex molecules, such as functional materials, with reactive species often causes side reactions. We will achieve gentle modification at desired sites by controlling the generation of reactive species using various external stimuli.
Lewis acid-mediated Suzuki-Miyaura cross-coupling reaction
The Suzuki-Miyaura cross-coupling (SMC) reaction is one of the most reliable carbon-carbon bond formation methods. This reaction requires adding a base to promote transmetalation with organoborons. On the other hand, a base also causes a reduction of efficiency by promoting competitive protodeborylation of organoboron compounds. In fact, some boronic acids can not be applied for the SMC reaction.
One of the simple strategies is avoiding the use of a base. In fact, SMC reaction with highly reactive aryldiazonium salts as coupling agents proceeds without an additional base. However, aromatic halides and other compounds commonly used in cross-coupling reactions are not applicable, probably because the palladium cationic intermediates are thermally unstable.
We found that some Lewis acids, such as electrophilic zinc complexes, promote SMC reaction with typical aryl halides.1 Mechanistic investigation using VT-NMR, solution XAS measurements, and theoretical analysis revealed the involvement of a cationic bimetallic complex bearing a certain thermostability. We assume that a reactive and thermally unstable cationic palladium complex gradually formed via the decomposition of the bimetallic complex, allowing for utilizing reactive species for the desired coupling reaction. Based on this speculation, we are expanding the utility of this mechanism by developing new transformations.
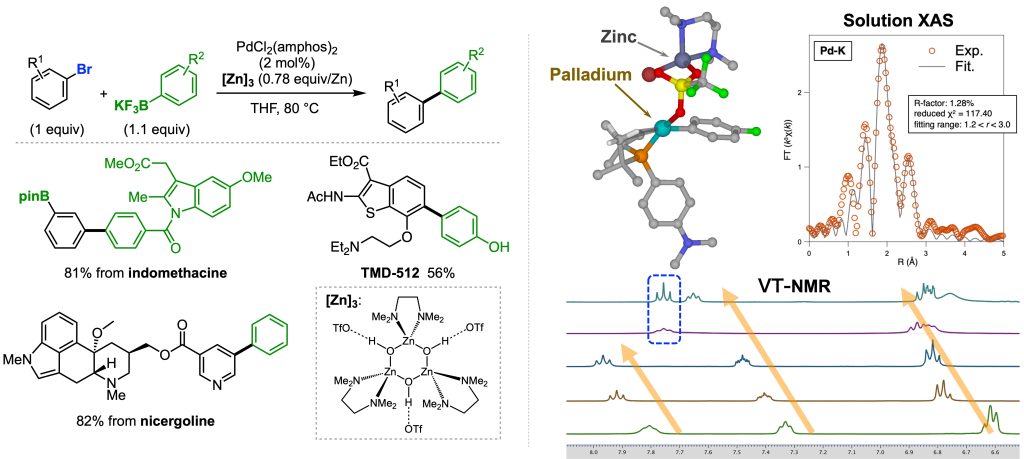
1. Nat. Catal. 2021, 4, 1080–1088.
(3) Creation of functional molecules that contribute to the development of drug discovery, medicine, and the life sciences
Functional molecules are demanded in various fields such as pharmaceutical, medical, and life sciences. However, their design and synthesis are not easy. Through collaboration with researchers in various fields, we efficiently create functional molecules that are truly in demand by applying our original and state-of-the-art organic reactions.
Development of molecular probes
We have been involved in the development of various molecular probes under collaboration projects. In particular, we have developed many PET probes for positron emission tomography (PET) imaging, a useful molecular imaging technique that can visualize the in vivo localization of molecules dynamically, three-dimensionally, and quantitatively, and is also applicable to humans. We have developed bioactive compounds, labeling reactions to introduce short-lived PET nuclides (half-lives: 20.4 min for carbon-11, 109.8 min for fluorine-18), and facile synthetic strategies for PET probes bearing complex chemical structures. We will continue to actively develop various functional molecular probes to meet the diverse needs of our collaborators.
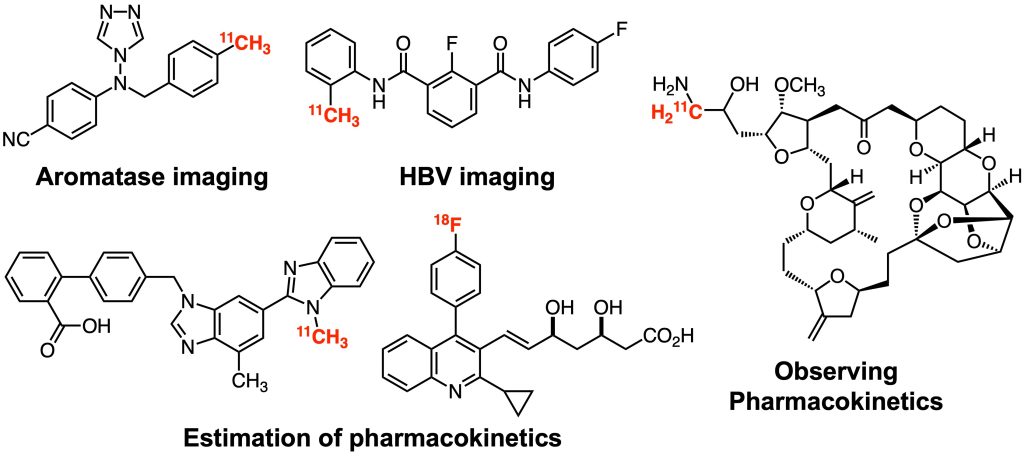
1. Org. Biomol. Chem. 2018, 16, 7711–7716.
2. Mol. Pharmaceutics 2020, 17, 1884–1898.
3. J. Neurosci. Res. 2020, 98, 2208–2218.
4. Drug Metab. Pharmacokinet. 2022, 44, 100449.
5. Bull. Chem. Soc. Jpn. 2023, 96, 283–290.
6. Compr. Psychiatry 2023, 123, 152381.
7. Angew. Chem. Int. Ed. 2023, Accepted Article.



